Muistuttajana taas englantilainen wikipedia, laitan tähän osan sen johdannosta opiskelijan merkkaamana.ja muokkaaman vastoin yliopiston kirjaston nimenomaista kieltoa.
Quantum mechanics (QM – also known as quantum physics, or quantum theory) is a branch of physics which deals with physical phenomena at nanoscopic scales where the action is on the order of the Planck constant.
It departs from classical mechanics primarily at the quantum realm of atomic and subatomic length scales.
Quantum mechanics provides a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter.
Quantum mechanics provides a substantially useful framework for many features of the modern periodic table of elements including the behavior of atoms during chemical bonding and has played a significant role in the development of many modern technologies.
In advanced topics of quantum mechanics, some of these behaviors are macroscopic (see macroscopic quantum phenomena) and emerge at only extreme (i.e., very low or very high) energies or temperatures (such as in the use of superconducting magnets). For example,
- the angular momentum of an electron bound to an atom or molecule is quantized.
- In contrast, the angular momentum of an unbound electron is not quantized.
In the context of quantum mechanics, the wave–particle duality of energy and matter and the uncertainty principle provide a unified view of the behavior of photons, electrons, and other atomic-scale objects.
The mathematical formulations of quantum mechanics are abstract.
A mathematical function, the wavefunction, provides information about the probability amplitude of position, momentum, and other physical properties of a particle. Mathematical manipulations of the wavefunction usually involve bra–ket notation which requires an understanding of complex numbers and linear functionals.
The wavefunction formulation treats the particle as a quantum harmonic oscillator, and the mathematics is akin to that describing acoustic resonance.
Many of the results of quantum mechanics are not easily visualized in terms of classical mechanics. For instance, in a quantum mechanical model the lowest energy state of a system, the ground state, is non-zero as opposed to a more "traditional" ground state with zero kinetic energy (all particles at rest). Instead of a traditional static, unchanging zero energy state, quantum mechanics allows for far more dynamic, chaotic possibilities, according to John Wheeler.
lue tämä wikipedian artikkeli kokonaisuudessaan
Kvanttimekaniikka ei siis ole jokamiehen hommaa. Sen syvempi ymmärtäminen edellyttää kompleksien numeroiden ja lineaaristen funktioiden hvyää tuntemusta. Tie kvanttimekaniikan ymmärtämiseen kulkee siis abstraktin matematiikan kautta, jota ihmisen aivojen on vaikea hahmottaa tässä meille tutussa todellisuudessa.
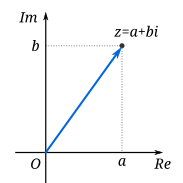 |
| A complex number can be visually represented as a pair of numbers (a, b) forming a vector on a diagram called an Argand diagram, representing the complex plane. "Re" is the real axis, "Im" is the imaginary axis, and i is the imaginary unit which satisfies the equation i2 = −1. kuva ja teksti wikipedia |
Ei kommentteja:
Lähetä kommentti